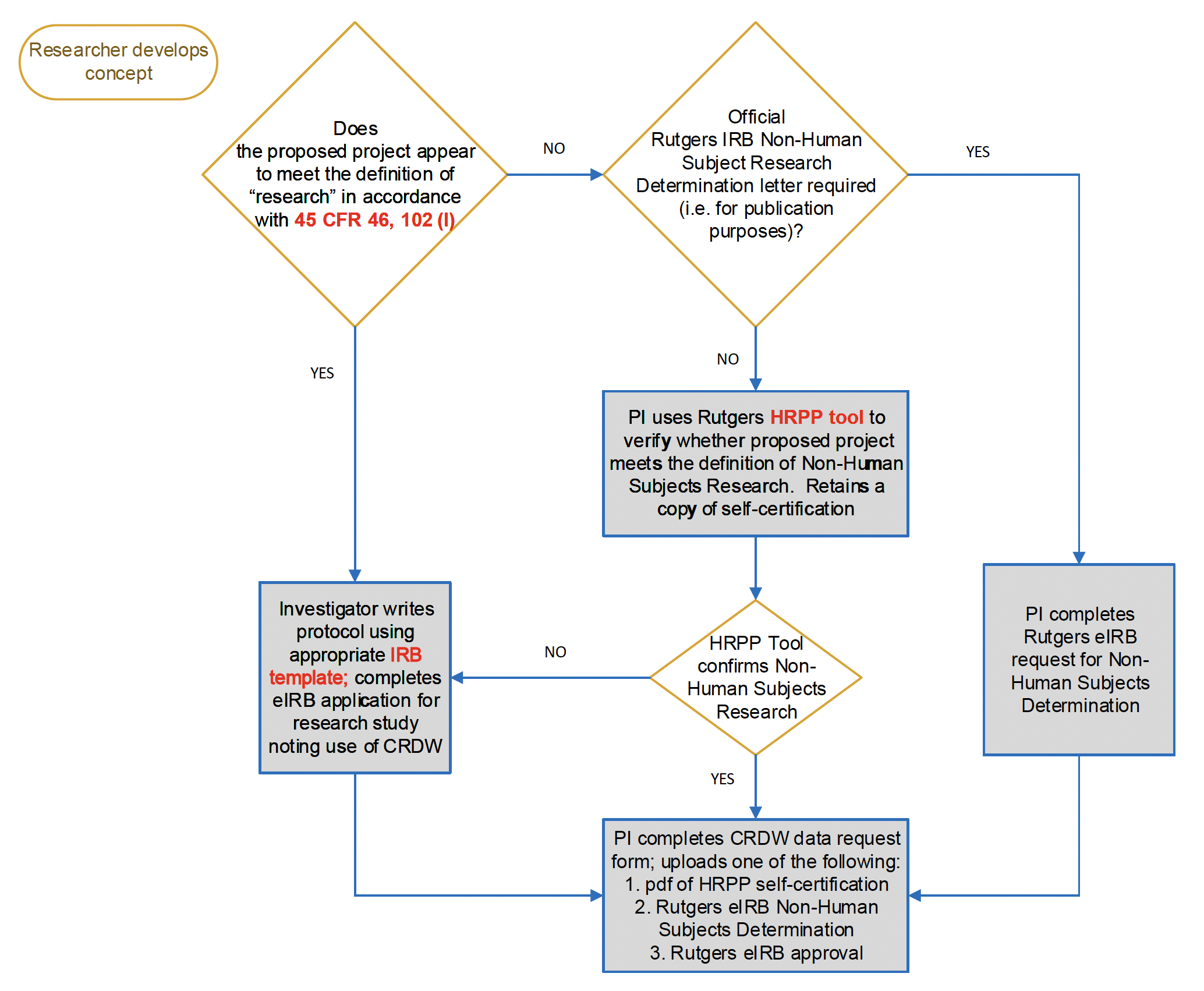
Institutional review board workflow
Institutional Review Board workflow
View text version below with acronyms expanded.
- Code of Federal Regulations
- Human Research Protection Program Toolkit
- Non-Human Research Self-Certification Tool
Institutional Review Board (IRB) workflow text version:
Researcher develops concept:
- 1. Does the proposed project appear to meet the definition of “research” in accordance with 45 CFR 46, 102 (I) (Code of Federal Regulations)
- If yes, proceed to process step 1a
- If no, proceed to process step 2
- a. Investigator writes protocol using appropriate IRB template; completes eIRB application for research study noting use of CRDW; once complete proceed to process step 1b
- b. PI (principal investigator) completes CRDW data request form; uploads one of the following: 1: PDF of HRPP (Human Research Protection Program) self-certification 2. Rutgers elRB Non-Human Subjects Determination 3. Rutgers elRB approval
- 2. Official Rutgers IRB Non-Human Subject Research Determination letter required (i.e. for publication purposes)?
- If yes, proceed to process step 2a
- If no, proceed to process step 2b
- a. PI completes Rutgers eIRB request for Non-Human Subjects Determination; once complete proceed to process step 1b
- b. PI uses Ruttgers HRPP tool to verify whether proposed project meets the definition of Non-Human Subjects Research. Retains a copy of self-certification; once complete proceed to process step 3
- 3. HRPP Tool confirms Non-Human Subjects Research
- If yes, proceed to process step 1b
- If no, proceed to process step 1a